Interatomic distance and lattice constant of NaCl. 1) The interaction potential energy between two ions of an ionic crystal can be approximated by the relation c) The distance $r_l$ where the electrostatic attraction energy is equal to the repulsive energy between ions. (The electron charge $e$ and the...Energy can be defined as the ability to do work. Physicists classify energy into several types: kinetic, potential, heat, sound, radiant energy (light, for example) Kinetic energy is possessed by a moving object by virtue of its motion. It equals the work done to accelerate the object to a particular velocity; it...The lattice energy is the amount of energy evolved when gaseous ions of opposite charges are brought together to form an ionic crystal composed of The concept of Lattice energ'y was originally developed for rock-salt structured and Sphalerite structure.The sphalerite structure is made up of zinc...Transmission: once the energy has been processed and turned into electricity, it is sent through overhead or underground wires from the plants to substations. There, transformers ensure sufficient electrical voltage. Substations tend to be above ground near to power plants, or on the outskirts of...Q. Which of the following statements about Geo constructs is true? Geo constructs can be used to block certain monster attacks. 7. Q. Which of the following cannot provide energy to charge an elemental burst? Interacting with the statue of seven.
UNIT 9. Text: "Energy".
Which statement is true about crystal lattice energy? a. It increases as the size of the ions increases. b. It increases as the charge on the ions decreases. c. It is the energy that ions absorb when they form a crystal. d. It is a measure of the strength of the bonds between ions.Lattice energy, the energy needed to completely separate an ionic solid, such as common table salt, into gaseous ions (also the energy released in the reverse process). Lattice energy is usually measured in kilojoules per mole (1 mole = 6.0221367 ¥ 1023).2) The reticular energy is the energy released when the solid Crystal isform from separate ions in the gaseous state. Always exothermic. 3) The enthalpy of the network depends directly on the size of the loads and conversely in the distance between the ions .2) The reticular energy is the energy released when the solid Crystal isform from separate ions in the gaseous state. Always exothermic. 3) The enthalpy of the network depends directly on the size of the loads and conversely in the distance between the ions . read more.

What is Lattice Energy(Crystal Energy) and how to determine lattice...
Definitions of lattice energy: * The energy released when one mole of a crystal is formed from gaseous ions.misterguch.brinkster.net/vocabulary.html * The energy for the reaction of the infinitely separated ions to give the solid. It measures the electrostatic interaction between the...Important Questions on Crystal Lattice And Unit Cell is available on Toppr. simple lattice. C. Unit cell. D. Crystal lattice. View Answer. The compound with the largest lattice energy would be: A. NaF.Lattice is commonly used to describe the crystal structures such as face centered cubic (FCC), body centered cubic (BCC) etc. The word lattice means a set of mathematical or imaginary points in space which satisfy translational symmetry. Lattice is commonly used to describe the crystal structures...1. Which of the following statements are true about work? Include all that apply. Work is a form of energy. d. TRUE - A kg•m2/s2 is a mass unit times a speed squared unit, making it a kinetic energy unit and equivalent to a Joule. e. FALSE - Work is not dependent on how rapidly the force displaces...The crystal lattice is used to describe the lattice of a real crystal. For example, in NaCl, a lattice point in a crystal lattice represents the position of a sodium ion or a chloride Bravais lattices are more mathematical and abstract than crystal lattices. They are pretty much the same as crystal lattices.
It increases as the dimensions of the ions will increase. It increases because the charge on the ions decreases. It is the energy that ions soak up after they shape a crystal. It is a measure of the power of the bonds between ions. read more
2) The reticular energy is the energy launched when the forged Crystal isform from separate ions within the gaseous state. Always exothermic. 3) The enthalpy of the network relies immediately on the dimension of the a lot and conversely within the distance between the ions . read extra
Solved: Which Of The Following Statements Concerning Latti ...

Which of the following compounds has the lowest boiling ...

X-ray : Wikis (The Full Wiki)

X-ray : Wikis (The Full Wiki)

X-ray : Wikis (The Full Wiki)

CHEM 112 Chemical Principles II | Clutch Prep

2.1.4 Periodic Potentials

Solid KF has a lattice energy of 804 kJmol and a heat of ...

Solved: Which One Of The Following Statements Is True? SH ...
Solved: Which Of The Following Has The Smallest Radius? A ...
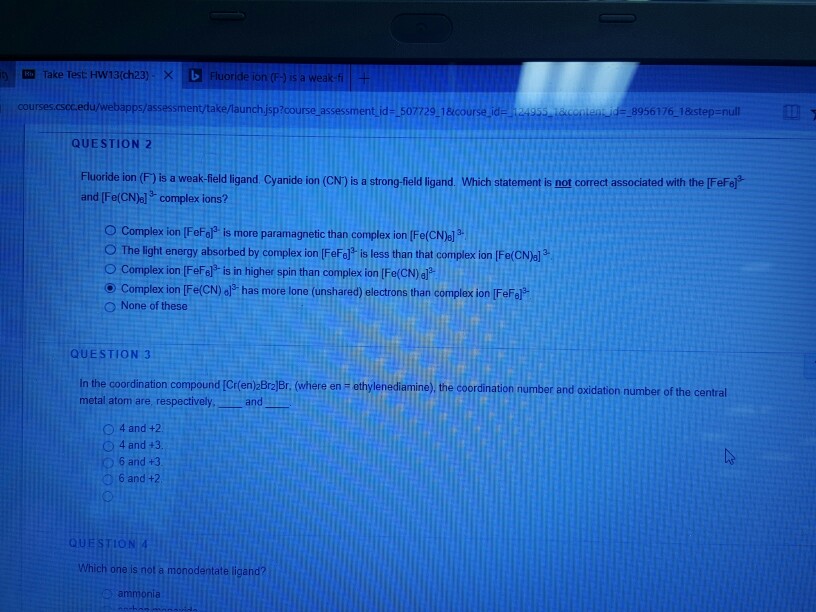
Solved: Fluoride Ion (F) Is A Weak-field Ligand. Cyanide I ...
savvy-chemist: Ionic Bonding (4) Lattice Energy and the ...

triple point

Evelin's Auto Care - Home | Facebook

X-ray : Wikis (The Full Wiki)

Chemistry Archive | February 27, 2017 | Chegg.com
Novel measurement techniques for visualizing 'live ...

Chemistry Archive | May 17, 2017 | Chegg.com
⚗️3. Which graph best matches a person walking away ...

Solid KF has a lattice energy of 804 kJmol and a heat of ...

Chapter05. Ionic Bond - Wildcat Chemistry Ionic Bonding ...







No comments:
Post a Comment