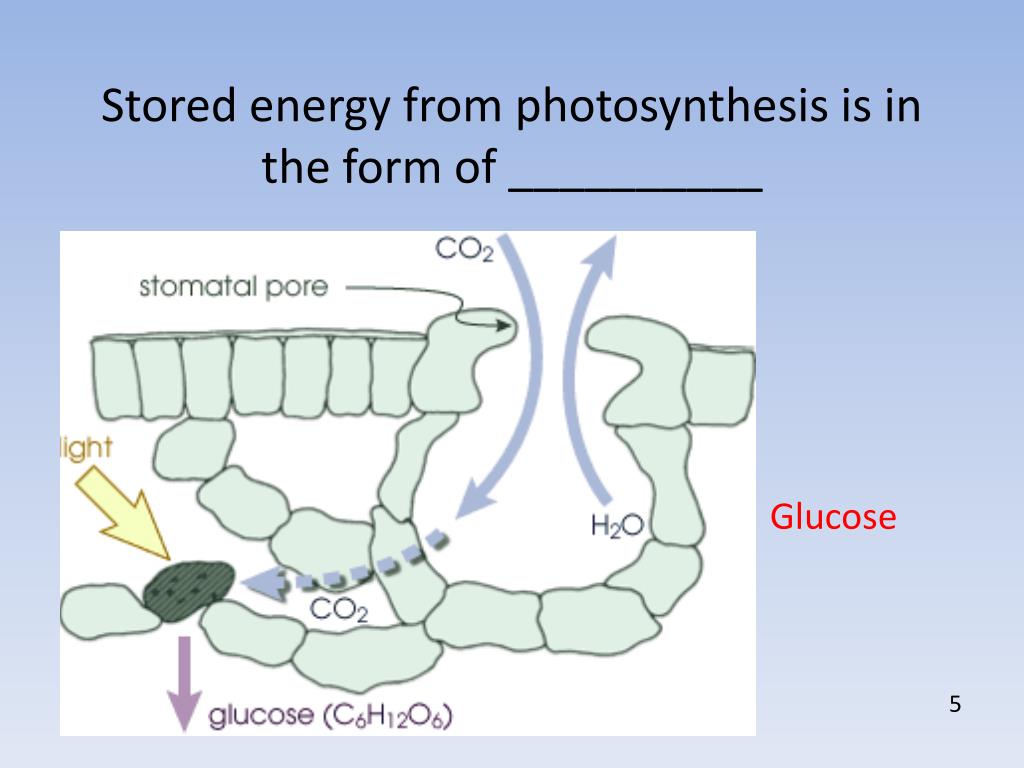
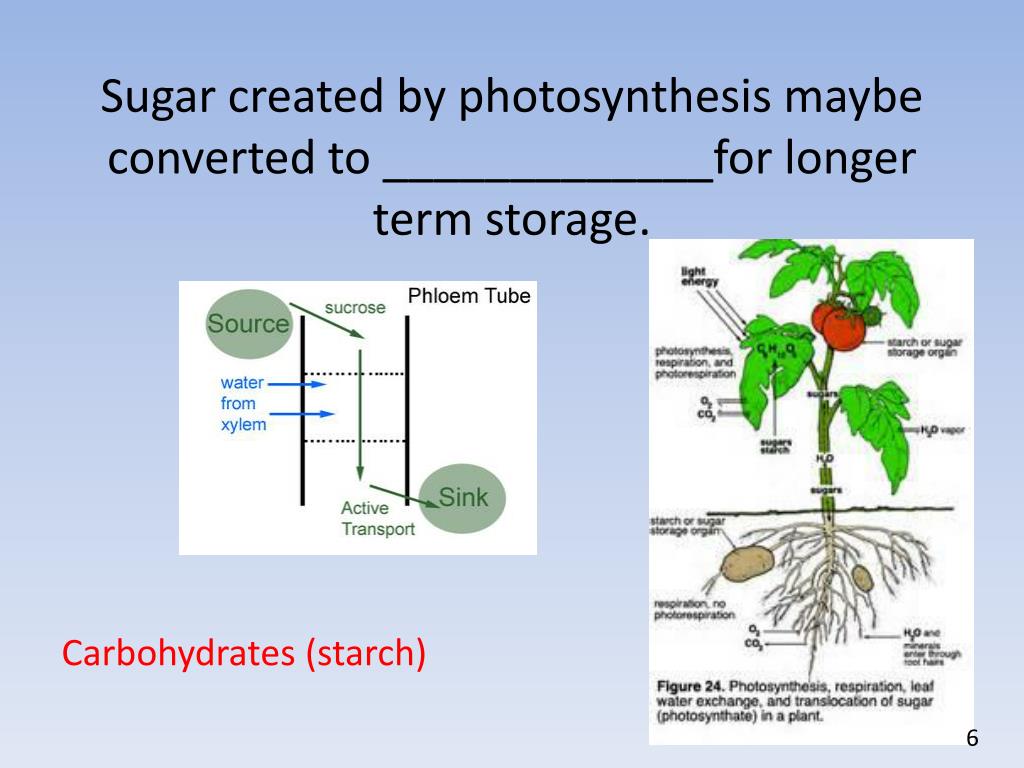
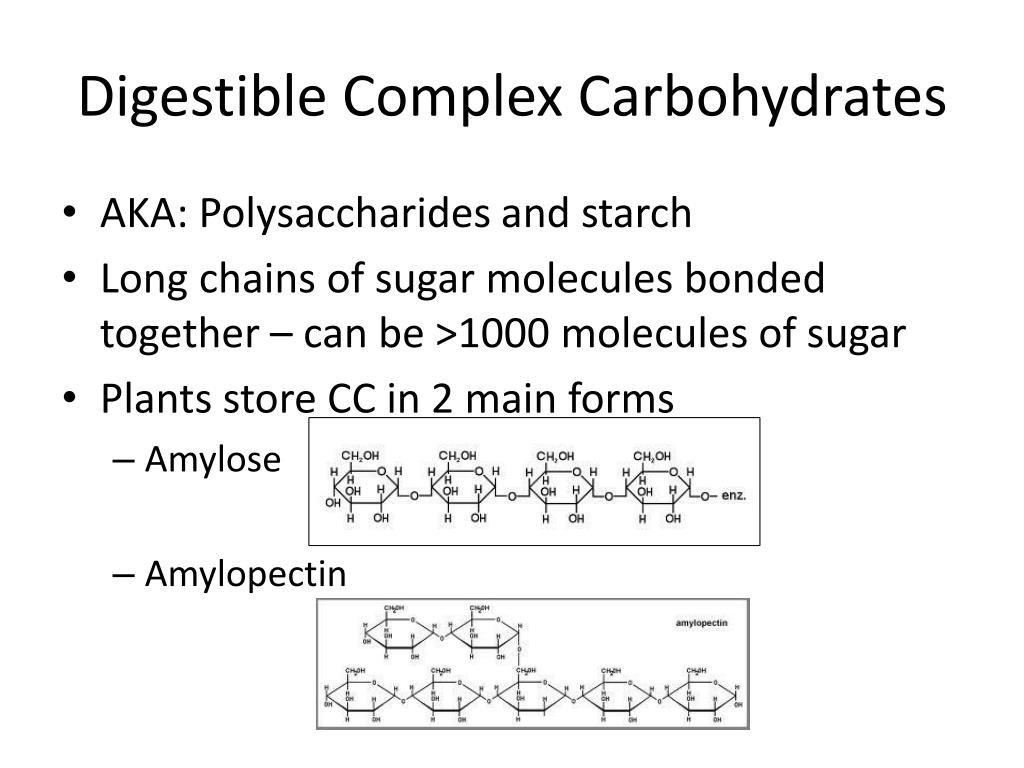
Glycogen is the analogue of starch, a less branched glucose polymer in plants, and is commonly referred to as animal starch, Glucose (Glc), a monosaccharide (or simple sugar), is an important carbohydrate in biology. The living cell uses it as a source of energy and metabolic intermediate.Carbohydrates in our diet include sugars and starches. The glucose molecule is small enough to be absorbed directly through the walls of the digestive system. In plant metabolism, the glucose produced by photosynthesis is converted into starch for storage, and cellulose, for cell wall synthesis.Starch is the stored form of sugars in plants and is made up of a mixture of amylose and amylopectin (both polymers of glucose). Plants are able to synthesize glucose, and the excess glucose, beyond the plant's immediate energy needs, is stored as starch in different plant parts, including roots and seeds.Carbohydrates: Starch and Glycogen in a Snap! Unlock the full A-level Biology course at http The key points covered in this video include: 1. Starch 2. Structure of Starch - Amylose 3. Structure of Starch - Amylopectin 4. Glycogen Starch In plants, the polysaccharide energy store is called starch.Carbohydrates are the one of the most important bio molecules present in the living organisms. The carbohydrates are the polymers of the sugars that are linked through the glycosidic bonds. The carbohydrate is stored in the form of starch in plants.
Structures of carbohydrates, proteins and lipids - Animal organisation...
Carbohydrates are the main source of energy for the body. They are the sugars, starches, and dietary fiber that occur in plant foods and dairy Foods high in carbohydrates include bread, pasta, beans, potatoes, rice, and cereals. Carbohydrates play several roles in living organisms, including...Fibre is found in the cell walls of foods that come from plants. Good sources of fibre include fruit and vegetables, wholegrain bread, wholewheat pasta, and A diet that is low in carbohydrates can lead to a lack of energy during exercise, early fatigue and delayed recovery. When is the best time to eat...A carbohydrate (/kɑːrboʊˈhaɪdreɪt/) is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen-oxygen atom ratio of 2:1 (as in water)...The corresponding polysaccharide in animals is called glycogen. Some starches can only be digested by the gut microbiota rather than our own body's If not used directly, the body converts glucose to glycogen, a polysaccharide like starch, which is stored in the liver and the muscles as a readily...

Reading: Structure and Function of Carbohydrates | Biology I
In plants and arthropods, carbohydrates from the skeletal structures, they also serve as food reserves in plants and animals. It is stored as glycogen in animals and starch in plants. Stored carbohydrates act as an energy source instead of proteins.Course Hero has all the homework and study help you need to succeed! We've got course-specific notes, study guides, and practice tests along with expert tutors. Find the best study resources around, tagged to your specific courses. Share your own to gain free Course Hero access.Glucose is the primary source of energy. It is the fuel most often burnt in cellular respiration. Excess glucose is polymerized and stored as glycogen in animals and starch in plants. Humans store glycogen in two locations; liver and muscles.The carbohydrates are the compounds which provide energy to living cells. They are compounds of carbon, hydrogen and oxygen with a ratio of two hydrogens for every oxygen atom. In animals, the equivalent of starches is glycogen, which can be stored in the muscles or in the liver for later use.To Maintain Homeostatis The Contraction Of Smooth Muscle In Blood Vessels (Vasoconstriction) Can Reduce The Flow Of Blood Through The Vessel. The World's First & Only Encyclopedia of Self Help, Self Improvement & Career Advice. 250+ Easy-to-Follow Guides 5000+ Proven Tips 13 Types of...
Jump to navigation Jump to go looking
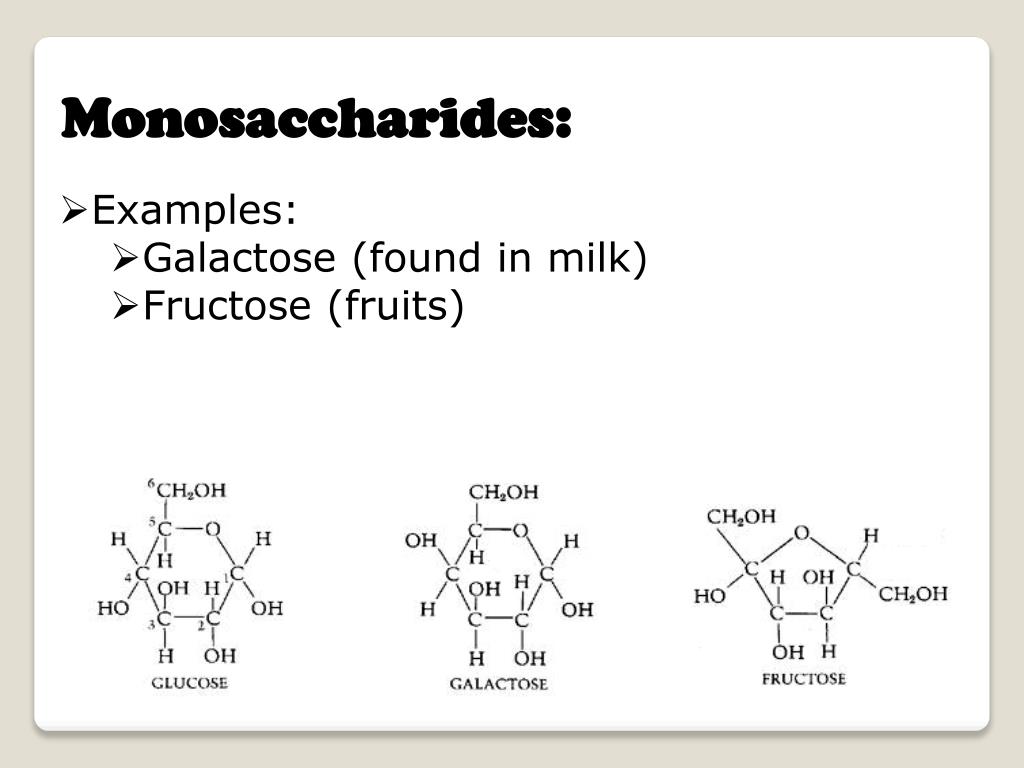
Lactose is a disaccharide found in animal milk. It is composed of a molecule of D-galactose and a molecule of D-glucose bonded by way of beta-1-4 glycosidic linkage.
A carbohydrate (/kɑːrboʊˈhaɪdreɪt/) is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, most often with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical components Cm(H2O)n (where m might or may not be other from n). However, now not all carbohydrates comply with this exact stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition robotically classified as carbohydrates (e.g. formaldehyde).
The term is most common in biochemistry, where it is a synonym of saccharide, a gaggle that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (decrease molecular weight) carbohydrates, are repeatedly known as sugars.[1] The phrase saccharide comes from the Greek phrase σάκχαρον (sákkharon), that means "sugar".[2] While the medical nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very frequently finish in the suffix -ose, which was in the beginning taken from glucose (gluekos), and is used for the majority sugars e.g. fructose (fruit sugar), sucrose (cane or beet sugar), ribose, amylose, lactose (milk sugar) and many others.
Carbohydrates carry out a lot of roles in dwelling organisms. Polysaccharides serve for the storage of power (e.g. starch and glycogen) and as structural parts (e.g. cellulose in vegetation and chitin in arthropods). The 5-carbon monosaccharide ribose is the most important part of coenzymes (e.g. ATP, FAD and NAD) and the backbone of the genetic molecule referred to as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives come with many other important biomolecules that play key roles in the immune system, fertilization, fighting pathogenesis, blood clotting, and building.[3]
Carbohydrates are central to vitamin and are discovered in a wide variety of herbal and processed meals. Starch is a polysaccharide. It is plentiful in cereals (wheat, maize, rice), potatoes, and processed food in response to cereal flour, akin to bread, pizza or pasta. Sugars appear in human diet basically as table sugar (sucrose, extracted from sugarcane or sugar beets), lactose (abundant in milk), glucose and fructose, either one of which happen naturally in honey, many end result, and some vegetables. Table sugar, milk, or honey are steadily added to beverages and lots of ready meals equivalent to jam, biscuits and desserts.
Cellulose, a polysaccharide discovered in the mobile walls of all vegetation, is considered one of the main elements of insoluble nutritional fiber. Although it is now not digestible, insoluble nutritional fiber is helping to maintain a wholesome digestive system[4] via easing defecation. Other polysaccharides contained in dietary fiber come with resistant starch and inulin, which feed some bacteria in the microbiota of the huge intestine, and are metabolized by these micro organism to yield short-chain fatty acids.[5][6]
Terminology
In scientific literature, the time period "carbohydrate" has many synonyms, like "sugar" (in the extensive sense), "saccharide", "ose",[2] "glucide",[7] "hydrate of carbon" or "polyhydroxy compounds with aldehyde or ketone". Some of these phrases, specially "carbohydrate" and "sugar", are also used with different meanings.
In meals science and in many casual contexts, the term "carbohydrate" continuously means any meals that is specifically rich in the advanced carbohydrate starch (corresponding to cereals, bread and pasta) or easy carbohydrates, reminiscent of sugar (discovered in sweet, jams, and desserts).
Often in lists of dietary data, akin to the USDA National Nutrient Database, the time period "carbohydrate" (or "carbohydrate by difference") is used for everything instead of water, protein, fats, ash, and ethanol.[8] This comprises chemical substances comparable to acetic or lactic acid, which aren't most often regarded as carbohydrates. It additionally comprises nutritional fiber which is a carbohydrate however which doesn't contribute much in the manner of food energy (kilocalories), even though it is often integrated in the calculation of total food power simply as although it were a sugar.
In the strict sense, "sugar" is implemented for sweet, soluble carbohydrates, lots of which are used in meals.
Structure
Formerly the title "carbohydrate" used to be used in chemistry for any compound with the formula Cm (H2O)n. Following this definition, some chemists considered formaldehyde (CH2O) to be the most straightforward carbohydrate,[9] while others claimed that name for glycolaldehyde.[10] Today, the time period is normally understood in the biochemistry sense, which excludes compounds with just one or two carbons and comprises many biological carbohydrates which deviate from this system. For instance, while the above consultant formulation would appear to capture the commonly identified carbohydrates, ubiquitous and considerable carbohydrates continuously deviate from this. For instance, carbohydrates frequently show chemical teams such as: N-acetyl (e.g. chitin), sulphate (e.g. glycosaminoglycans), carboxylic acid (e.g. sialic acid) and deoxy changes (e.g. fucose and sialic acid).
Natural saccharides are most often constructed of easy carbohydrates referred to as monosaccharides with basic formulation (CH2O)n the place n is 3 or extra. A typical monosaccharide has the structure H–(CHOH)x(C=O)–(CHOH)y–H, that is, an aldehyde or ketone with many hydroxyl teams added, usually one on every carbon atom that is now not a part of the aldehyde or ketone practical staff. Examples of monosaccharides are glucose, fructose, and glyceraldehydes. However, some organic elements often called "monosaccharides" don't conform to this formulation (e.g. uronic acids and deoxy-sugars comparable to fucose) and there are many chemicals that do conform to this components but are not regarded as to be monosaccharides (e.g. formaldehyde CH2O and inositol (CH2O)6).[11]
The open-chain form of a monosaccharide steadily coexists with a closed ring form where the aldehyde/ketone carbonyl team carbon (C=O) and hydroxyl workforce (–OH) react forming a hemiacetal with a brand new C–O–C bridge.
Monosaccharides may also be connected in combination into what are called polysaccharides (or oligosaccharides) in a large variety of tactics. Many carbohydrates contain one or more modified monosaccharide units that experience had a number of groups replaced or got rid of. For instance, deoxyribose, an element of DNA, is a changed model of ribose; chitin is composed of repeating units of N-acetyl glucosamine, a nitrogen-containing form of glucose.
Division
Carbohydrates are polyhydroxy aldehydes, ketones, alcohols, acids, their easy derivatives and their polymers having linkages of the acetal type. They could also be classified in step with their stage of polymerization, and could also be divided to start with into 3 essential teams, namely sugars, oligosaccharides and polysaccharides[12]
The main dietary carbohydrates Class(degree of polymerization) Subgroup Components Sugars (1–2) Monosaccharides Glucose, galactose, fructose, xylose Disaccharides Sucrose, lactose, maltose, isomaltulose, trehalose Polyols Sorbitol, mannitol Oligosaccharides (3–9) Malto-oligosaccharides Maltodextrins Other oligosaccharides Raffinose, stachyose, fructo-oligosaccharides Polysaccharides (>9) Starch Amylose, amylopectin, changed starches Non-starch polysaccharides Glycogen, Cellulose, Hemicellulose, Pectins, HydrocolloidsMonosaccharides
Main article: Monosaccharide D-glucose is an aldohexose with the formula (C·H2O)6. The crimson atoms spotlight the aldehyde team and the blue atoms highlight the asymmetric heart furthest from the aldehyde; because this -OH is on the appropriate of the Fischer projection, this is a D sugar.Monosaccharides are the simplest carbohydrates in that they cannot be hydrolyzed to smaller carbohydrates. They are aldehydes or ketones with two or more hydroxyl teams. The common chemical formulation of an unmodified monosaccharide is (C•H2O)n, actually a "carbon hydrate". Monosaccharides are vital gasoline molecules in addition to building blocks for nucleic acids. The smallest monosaccharides, for which n=3, are dihydroxyacetone and D- and L-glyceraldehydes.
Classification of monosaccharides
The α and β anomers of glucose. Note the position of the hydroxyl workforce (pink or inexperienced) on the anomeric carbon relative to the CH2OH workforce sure to carbon 5: they either have an identical absolute configurations (R,R or S,S) (α), or opposite absolute configurations (R,S or S,R) (β).[13]
Monosaccharides are categorised in line with 3 other traits: the placement of its carbonyl staff, the selection of carbon atoms it incorporates, and its chiral handedness. If the carbonyl crew is an aldehyde, the monosaccharide is an aldose; if the carbonyl crew is a ketone, the monosaccharide is a ketose. Monosaccharides with 3 carbon atoms are referred to as trioses, those with four are known as tetroses, 5 are called pentoses, six are hexoses, and so forth.[14] These two methods of classification are continuously combined. For instance, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone).
Each carbon atom bearing a hydroxyl group (-OH), with the exception of the first and ultimate carbons, are asymmetric, making them stereo centers with two imaginable configurations every (R or S). Because of this asymmetry, plenty of isomers might exist for any given monosaccharide components. Using Le Bel-van't Hoff rule, the aldohexose D-glucose, as an example, has the components (C·H2O)6, of which 4 of its six carbons atoms are stereogenic, making D-glucose one in all 24=Sixteen imaginable stereoisomers. In the case of glyceraldehydes, an aldotriose, there is one pair of possible stereoisomers, which can be enantiomers and epimers. 1, 3-dihydroxyacetone, the ketose similar to the aldose glyceraldehydes, is a symmetric molecule with no stereo facilities. The task of D or L is made in keeping with the orientation of the uneven carbon furthest from the carbonyl crew: in a standard Fischer projection if the hydroxyl staff is on the right the molecule is a D sugar, otherwise it is an L sugar. The "D-" and "L-" prefixes should now not be puzzled with "d-" or "l-", which indicate the route that the sugar rotates plane polarized light. This usage of "d-" and "l-" is no longer followed in carbohydrate chemistry.[15]
Ring-straight chain isomerism Glucose can exist in each a straight-chain and ring form.The aldehyde or ketone crew of a straight-chain monosaccharide will react reversibly with a hydroxyl team on a special carbon atom to form a hemiacetal or hemiketal, forming a heterocyclic ring with an oxygen bridge between two carbon atoms. Rings with five and six atoms are known as furanose and pyranose paperwork, respectively, and exist in equilibrium with the straight-chain shape.[16]
During the conversion from straight-chain shape to the cyclic form, the carbon atom containing the carbonyl oxygen, known as the anomeric carbon, turns into a stereogenic heart with two conceivable configurations: The oxygen atom may take a position both above or beneath the aircraft of the ring. The resulting imaginable pair of stereoisomers is referred to as anomers. In the α anomer, the -OH substituent on the anomeric carbon rests on the reverse facet (trans) of the ring from the CH2OH facet branch. The choice shape, in which the CH2OH substituent and the anomeric hydroxyl are on the similar facet (cis) of the plane of the ring, is referred to as the β anomer.
Use in dwelling organismsMonosaccharides are the major fuel supply for metabolism, getting used both as an power supply (glucose being the most necessary in nature) and in biosynthesis. When monosaccharides are not straight away needed by many cells, they are often converted to extra space-efficient forms, frequently polysaccharides. In many animals, together with humans, this storage form is glycogen, particularly in liver and muscle cells. In crops, starch is used for the similar goal. The maximum ample carbohydrate, cellulose, is a structural part of the mobile wall of vegetation and plenty of sorts of algae. Ribose is an element of RNA. Deoxyribose is a component of DNA. Lyxose is an element of lyxoflavin found in the human middle.[17]Ribulose and xylulose happen in the pentose phosphate pathway. Galactose, a component of milk sugar lactose, is found in galactolipids in plant cellular membranes and in glycoproteins in many tissues. Mannose occurs in human metabolism, particularly in the glycosylation of sure proteins. Fructose, or fruit sugar, is found in many vegetation and people, it is metabolized in the liver, absorbed at once into the intestines all over digestion, and found in semen. Trehalose, a major sugar of insects, is unexpectedly hydrolyzed into two glucose molecules to beef up steady flight.
Disaccharides
Sucrose, often referred to as desk sugar, is a common disaccharide. It is composed of two monosaccharides: D-glucose (left) and D-fructose (right). Main article: DisaccharideTwo joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples come with sucrose and lactose. They are composed of 2 monosaccharide units certain together by way of a covalent bond referred to as a glycosidic linkage shaped by way of a dehydration reaction, ensuing in the lack of a hydrogen atom from one monosaccharide and a hydroxyl staff from the different. The formulation of unmodified disaccharides is C12H22O11. Although there are a lot of kinds of disaccharides, a handful of disaccharides are specifically notable.
Sucrose, pictured to the appropriate, is the most abundant disaccharide, and the major form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule. The systematic title for sucrose, O-α-D-glucopyranosyl-(1→2)-D-fructofuranoside, signifies 4 issues:
Its monosaccharides: glucose and fructose Their ring types: glucose is a pyranose and fructose is a furanose How they are related together: the oxygen on carbon number one (C1) of α-D-glucose is linked to the C2 of D-fructose. The -oside suffix indicates that the anomeric carbon of each monosaccharides participates in the glycosidic bond.Lactose, a disaccharide composed of 1 D-galactose molecule and one D-glucose molecule, occurs naturally in mammalian milk. The systematic identify for lactose is O-β-D-galactopyranosyl-(1→4)-D-glucopyranose. Other notable disaccharides include maltose (two D-glucoses related α-1,4) and cellobiose (two D-glucoses related β-1,4). Disaccharides will also be labeled into two sorts: lowering and non-reducing disaccharides. If the functional staff is provide in bonding with any other sugar unit, it is referred to as a decreasing disaccharide or biose.
Nutrition
Grain merchandise: rich sources of carbohydratesCarbohydrate fed on in meals yields 3.87 kilocalories of energy consistent with gram for easy sugars,[18] and three.57 to 4.12 kilocalories consistent with gram for complex carbohydrate in most other foods.[19] Relatively high levels of carbohydrate are associated with processed foods or delicate meals constructed from vegetation, together with chocolates, cookies and candy, table sugar, honey, comfortable drinks, breads and crackers, jams and fruit products, pastas and breakfast cereals. Lower quantities of carbohydrate are generally related to unrefined foods, together with beans, tubers, rice, and unrefined fruit.[20] Animal-based foods most often have the lowest carbohydrate levels, even supposing milk does comprise a excessive share of lactose.
Organisms most often can't metabolize all sorts of carbohydrate to yield energy. Glucose is a nearly common and obtainable supply of power. Many organisms even have the skill to metabolize other monosaccharides and disaccharides but glucose is incessantly metabolized first. In Escherichia coli, for example, the lac operon will express enzymes for the digestion of lactose when it is present, but when both lactose and glucose are provide the lac operon is repressed, ensuing in the glucose being used first (see: Diauxie). Polysaccharides also are common sources of power. Many organisms can simply ruin down starches into glucose; most organisms, then again, can't metabolize cellulose or other polysaccharides like chitin and arabinoxylans. These carbohydrate varieties may also be metabolized by means of some bacteria and protists. Ruminants and termites, as an example, use microorganisms to process cellulose. Even though these complex carbohydrates don't seem to be very digestible, they constitute crucial nutritional element for people, referred to as nutritional fiber. Fiber complements digestion, among different advantages.[21]
The Institute of Medicine recommends that American and Canadian adults get between Forty five and 65% of dietary power from whole-grain carbohydrates.[22] The Food and Agriculture Organization and World Health Organization jointly recommend that national nutritional tips set a objective of 55–75% of general energy from carbohydrates, however handiest 10% directly from sugars (their time period for simple carbohydrates).[23] A 2017 Cochrane Systematic Review concluded that there was insufficient proof to improve the declare that whole grain diets can impact heart problems.[24]
ClassificationNutritionists ceaselessly confer with carbohydrates as both simple or complicated. However, the exact difference between these teams can also be ambiguous. The term complex carbohydrate was first used in the U.S. Senate Select Committee on Nutrition and Human Needs e-newsletter Dietary Goals for the United States (1977) where it was meant to differentiate sugars from other carbohydrates (that have been perceived to be nutritionally superior).[25] However, the record put "fruit, vegetables and whole-grains" in the advanced carbohydrate column, despite the indisputable fact that those may include sugars as well as polysaccharides. This confusion persists as today some nutritionists use the term complex carbohydrate to consult with any kind of digestible saccharide present in a complete food, where fiber, vitamins and minerals are also discovered (versus processed carbohydrates, which give energy but few different vitamins). The usual usage, on the other hand, is to categorise carbohydrates chemically: simple if they are sugars (monosaccharides and disaccharides) and sophisticated if they are polysaccharides (or oligosaccharides).[26]
In any case, the simple vs. complicated chemical difference has little worth for determining the nutritional quality of carbohydrates.[26] Some simple carbohydrates (e.g. fructose) raise blood glucose impulsively, while some complex carbohydrates (starches), elevate blood sugar slowly. The pace of digestion is decided through various factors together with which other vitamins are fed on with the carbohydrate, how the food is prepared, person variations in metabolism, and the chemistry of the carbohydrate.[27] Carbohydrates are occasionally divided into "available carbohydrates", which might be absorbed in the small gut and "unavailable carbohydrates", which cross to the large gut, the place they are matter to fermentation by way of the gastrointestinal microbiota.[28]
The USDA's Dietary Guidelines for Americans 2010 name for moderate- to high-carbohydrate consumption from a balanced vitamin that comes with six one-ounce servings of grain foods every day, no less than part from whole grain sources and the rest from enriched.[29]
The glycemic index (GI) and glycemic load ideas have been advanced to symbolize meals conduct all through human digestion. They rank carbohydrate-rich foods in response to the rapidity and magnitude in their impact on blood glucose ranges. Glycemic index is a measure of how quickly meals glucose is absorbed, while glycemic load is a measure of the total absorbable glucose in foods. The insulin index is a similar, more moderen classification manner that ranks foods in keeping with their effects on blood insulin ranges, which can be caused by glucose (or starch) and some amino acids in food.
Health results of nutritional carbohydrate restriction Main article: Low-carbohydrate dietLow-carbohydrate diets might omit the well being advantages – akin to greater intake of nutritional fiber – afforded through top of the range carbohydrates discovered in legumes and pulses, whole grains, culmination, and greens.[30][31] Disadvantages of the nutrition would possibly come with halitosis, headache and constipation, and in general the doable opposed results of carbohydrate-restricted diets are under-researched, in particular for imaginable risks of osteoporosis and cancer occurrence.[32]
Carbohydrate-restricted diets will also be as effective as low-fat diets in serving to achieve weight reduction over the quick time period when general calorie intake is reduced.[33] An Endocrine Society scientific statement stated that "when calorie intake is held constant [...] body-fat accumulation does not appear to be affected by even very pronounced changes in the amount of fat vs carbohydrate in the diet."[33] In the long run, efficient weight loss or maintenance depends upon calorie restriction,[33] no longer the ratio of macronutrients in a diet.[34] The reasoning of vitamin advocates that carbohydrates cause undue fat accumulation by way of increasing blood insulin ranges, and that low-carbohydrate diets have a "metabolic advantage", is now not supported via scientific evidence.[33][35] Further, it is now not transparent how low-carbohydrate eating plan affects cardiovascular health, despite the fact that two reviews showed that carbohydrate restriction may make stronger lipid markers of heart problems possibility.[36][37]
Carbohydrate-restricted diets are no more effective than a standard nutritious diet in fighting the onset of sort 2 diabetes, however for other folks with sort 2 diabetes, they are a viable choice for reducing weight or serving to with glycemic keep watch over.[38][39][40] There is limited proof to toughen regimen use of low-carbohydrate dieting in managing type 1 diabetes.[41] The American Diabetes Association recommends that folks with diabetes must undertake a usually nutritious diet, relatively than a nutrition excited about carbohydrate or other macronutrients.[40]
An extreme type of low-carbohydrate nutrition – the ketogenic vitamin – is established as a clinical vitamin for treating epilepsy.[42] Through celebrity endorsement all over the early 21st century, it changed into a fad diet as a method of weight loss, however with risks of undesirable unwanted effects, equivalent to low energy levels and higher hunger, insomnia, nausea, and gastrointestinal discomfort.[42] The British Dietetic Association named it certainly one of the "top 5 worst celeb diets to avoid in 2018".[42]
Metabolism
Main article: Carbohydrate metabolismCarbohydrate metabolism is the series of biochemical processes responsible for the formation, breakdown and interconversion of carbohydrates in living organisms.
The most important carbohydrate is glucose, a easy sugar (monosaccharide) that is metabolized through nearly all identified organisms. Glucose and other carbohydrates are a part of all kinds of metabolic pathways throughout species: vegetation synthesize carbohydrates from carbon dioxide and water by photosynthesis storing the absorbed energy internally, often in the form of starch or lipids. Plant elements are ate up via animals and fungi, and used as gas for cellular respiration. Oxidation of 1 gram of carbohydrate yields roughly 16 kJ (4 kcal) of power, while the oxidation of 1 gram of lipids yields about 38 kJ (9 kcal). The human frame retail outlets between Three hundred and 500 g of carbohydrates relying on frame weight, with the skeletal muscle contributing to a large portion of the storage.[43] Energy acquired from metabolism (e.g., oxidation of glucose) is usually stored briefly inside cells in the type of ATP.[44] Organisms able to anaerobic and cardio breathing metabolize glucose and oxygen (cardio) to liberate energy, with carbon dioxide and water as byproducts.
CatabolismCatabolism is the metabolic response which cells go through to wreck down larger molecules, extracting energy. There are two major metabolic pathways of monosaccharide catabolism: glycolysis and the citric acid cycle.
In glycolysis, oligo- and polysaccharides are cleaved first to smaller monosaccharides through enzymes called glycoside hydrolases. The monosaccharide devices can then input into monosaccharide catabolism. A 2 ATP investment is required in the early steps of glycolysis to phosphorylate Glucose to Glucose 6-Phosphate (G6P) and Fructose 6-Phosphate (F6P) to Fructose 1,6-biphosphate (FBP), thereby pushing the response ahead irreversibly.[43] In some cases, as with people, not all carbohydrate sorts are usable as the digestive and metabolic enzymes important are not provide.
Carbohydrate chemistry
Carbohydrate chemistry is a large and economically important branch of natural chemistry. Some of the major natural reactions that involve carbohydrates are:
Carbohydrate acetalisation Cyanohydrin reaction Lobry de Bruyn–Van Ekenstein transformation Amadori rearrangement Nef reaction Wohl degradation Koenigs–Knorr response Carbohydrate digestionSee also
Bioplastic Fermentation Glycobiology Glycoinformatics Glycolipid Glycome Glycomics Glycosyl Macromolecule Low-carbohydrate nutrition Pentose phosphate pathway Photosynthesis Resistant starch Saccharic acid Carbohydrate NMRReferences
^ .mw-parser-output cite.citationfont-style:inherit.mw-parser-output .quotation qquotes:"\"""\"""'""'".mw-parser-output .id-lock-free a,.mw-parser-output .citation .cs1-lock-free abackground:linear-gradient(transparent,clear),url("//upload.wikimedia.org/wikipedia/commons/6/65/Lock-green.svg")correct 0.1em center/9px no-repeat.mw-parser-output .id-lock-limited a,.mw-parser-output .id-lock-registration a,.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:linear-gradient(clear,transparent),url("//upload.wikimedia.org/wikipedia/commons/d/d6/Lock-gray-alt-2.svg")appropriate 0.1em heart/9px no-repeat.mw-parser-output .id-lock-subscription a,.mw-parser-output .citation .cs1-lock-subscription abackground:linear-gradient(transparent,clear),url("//upload.wikimedia.org/wikipedia/commons/a/aa/Lock-red-alt-2.svg")correct 0.1em middle/9px no-repeat.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:linear-gradient(transparent,clear),url("//upload.wikimedia.org/wikipedia/commons/4/4c/Wikisource-logo.svg")appropriate 0.1em middle/12px no-repeat.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:none;padding:inherit.mw-parser-output .cs1-hidden-errorshow:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;colour:#33aa33;margin-left:0.3em.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em.mw-parser-output .quotation .mw-selflinkfont-weight:inheritFlitsch SL, Ulijn RV (January 2003). "Sugars tied to the spot". Nature. 421 (6920): 219–20. Bibcode:2003Natur.421..219F. doi:10.1038/421219a. PMID 12529622. S2CID 4421938. ^ a b Avenas P (2012). "Etymology of main polysaccharide names" (PDF). In Navard P (ed.). The European Polysaccharide Network of Excellence (EPNOE). Wien: Springer-Verlag. ^ Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1993). Human Biology and Health. Englewood Cliffs, New Jersey: Prentice Hall. pp. 52–59. ISBN 978-0-13-981176-0. ^ USDA National Nutrient Database, 2015, p. 14 ^ Cummings, John H. (2001). The Effect of Dietary Fiber on Fecal Weight and Composition (third ed.). Boca Raton, Florida: CRC Press. p. 184. ISBN 978-0-8493-2387-4. ^ Byrne CS, Chambers ES, Morrison DJ, Frost G (September 2015). "The role of short chain fatty acids in appetite regulation and energy homeostasis". International Journal of Obesity. 39 (9): 1331–8. doi:10.1038/ijo.2015.84. PMC 4564526. PMID 25971927. ^ Fearon WF (1949). Introduction to Biochemistry (second ed.). London: Heinemann. ISBN 9781483225395. ^ USDA National Nutrient Database, 2015, p. 13 ^ Coulter JM, Barnes CR, Cowles HC (1930). A Textbook of Botany for Colleges and Universities. ISBN 9781113909954. ^ Burtis CA, Ashwood ER, Tietz NW (2000). Tietz fundamentals of scientific chemistry. ISBN 9780721686349. ^ Matthews CE, Van Holde KE, Ahern KG (1999). Biochemistry (third ed.). Benjamin Cummings. ISBN 978-0-8053-3066-3. ^ "Chapter 1 – The role of carbohydrates in nutrition". Carbohydrates in human nutrition. FAO Food and Nutrition Paper – 66. Food and Agriculture Organization of the United Nations. ^ Bertozzi CR, Rabuka D (2017). "Structural Basis of Glycan Diversity". Essentials of Glycobiology (3rd ed.). Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. ISBN 978-1-621821-32-8. ^ Campbell NA, Williamson B, Heyden RJ (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 978-0-13-250882-7. ^ Pigman W, Horton D (1972). "Chapter 1: Stereochemistry of the Monosaccharides". In Pigman and Horton (ed.). The Carbohydrates: Chemistry and Biochemistry Vol 1A (2nd ed.). San Diego: Academic Press. pp. 1–67. ISBN 9780323138338. ^ Pigman W, Anet E (1972). "Chapter 4: Mutarotations and Actions of Acids and Bases". In Pigman and Horton (ed.). The Carbohydrates: Chemistry and Biochemistry Vol 1A (2d ed.). San Diego: Academic Press. pp. 165–94. ISBN 9780323138338. ^ "lyxoflavin". Merriam-Webster. ^ "Show Foods". usda.gov. ^ "Calculation of the Energy Content of Foods – Energy Conversion Factors". fao.org. ^ "Carbohydrate reference list" (PDF). www.diabetes.org.uk. Retrieved October 30, 2016. ^ Pichon L, Huneau JF, Fromentin G, Tomé D (May 2006). "A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats". The Journal of Nutrition. 136 (5): 1256–60. doi:10.1093/jn/136.5.1256. PMID 16614413. ^ Food and Nutrition Board (2002/2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids Archived February 10, 2007, at Archive.nowadays. Washington, D.C.: The National Academies Press. Page 769 Archived September 12, 2006, at the Wayback Machine. ISBN 0-309-08537-3. ^ Joint WHO/FAO knowledgeable session (2003). [1] (PDF). Geneva: World Health Organization. pp. 55–56. ISBN 92-4-120916-X. ^ Kelly SA, Hartley L, Loveman E, Colquitt JL, Jones HM, Al-Khudairy L, Clar C, Germanò R, Lunn HR, Frost G, Rees Okay (2017). "Whole grain cereals for the primary or secondary prevention of cardiovascular disease" (PDF). The Cochrane Database of Systematic Reviews. 8: CD005051. doi:10.1002/14651858.CD005051.pub3. PMC 6484378. PMID 28836672. ^ Joint WHO/FAO expert session (1998), Carbohydrates in human diet, chapter 1. ISBN 92-5-104114-8. ^ a b "Carbohydrates". The Nutrition Source. Harvard School of Public Health. September 18, 2012. Retrieved April 3, 2013. ^ Jenkins DJ, Jenkins AL, Wolever TM, Thompson LH, Rao AV (February 1986). "Simple and complex carbohydrates". Nutrition Reviews. 44 (2): 44–9. doi:10.1111/j.1753-4887.1986.tb07585.x. PMID 3703387. ^ Hedley, C. L. (2001). Carbohydrates in Grain Legume Seeds: Improving Nutritional Quality and Agronomic Characteristics. CABI. p. 79. ISBN 978-0-85199-944-9. ^ DHHS and USDA, Dietary Guidelines for Americans 2010 Archived August 20, 2014, at the Wayback Machine. ^ Seidelmann, Sara B; Claggett, Brian; Cheng, Susan; Henglin, Mir; Shah, Amil; Steffen, Lyn M; Folsom, Aaron R; Rimm, Eric B; Willett, Walter C; Solomon, Scott D (2018). "Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis". The Lancet. Public Health (Meta-analysis). 3 (9): e419–e428. doi:10.1016/s2468-2667(18)30135-x. ISSN 2468-2667. PMC 6339822. PMID 30122560. ^ Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L (January 10, 2019). "Carbohydrate quality and human health: a series of systematic reviews and meta-analyses" (PDF). Lancet (Review). 393 (10170): 434–445. doi:10.1016/S0140-6736(18)31809-9. PMID 30638909. S2CID 58632705. ^ Churuangsuk C, Kherouf M, Combet E, Lean M (2018). "Low-carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews" (PDF). Obesity Reviews (Systematic assessment). 19 (12): 1700–1718. doi:10.1111/obr.12744. PMID 30194696. S2CID 52174104. ^ a b c d Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. (2017). "Obesity Pathogenesis: An Endocrine Society Scientific Statement". Endocrine Reviews. 38 (4): 267–296. doi:10.1210/er.2017-00111. PMC 5546881. PMID 28898979. ^ Butryn ML, Clark VL, Coletta MC (2012). Akabas SR, et al. (eds.). Behavioral approaches to the treatment of obesity. Textbook of Obesity. John Wiley & Sons. p. 259. ISBN 978-0-470-65588-7. Taken in combination, these findings indicate that calorie intake, not macronutrient composition, determines long-term weight loss repairs. ^ Hall KD (2017). "A review of the carbohydrate-insulin model of obesity". European Journal of Clinical Nutrition (Review). 71 (3): 323–326. doi:10.1038/ejcn.2016.260. PMID 28074888. S2CID 54484172. ^ Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K (February 2016). "Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials". The British Journal of Nutrition. 115 (3): 466–79. doi:10.1017/S0007114515004699. PMID 26768850. S2CID 21670516. ^ Gjuladin-Hellon T, Davies IG, Penson P, Amiri Baghbadorani R (2019). "Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: a systematic review and meta-analysis" (PDF). Nutrition Reviews (Systematic evaluate). 77 (3): 161–180. doi:10.1093/nutrit/nuy049. PMID 30544168. S2CID 56488132. ^ Brouns F (2018). "Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable?". Eur J Nutr (Review). 57 (4): 1301–1312. doi:10.1007/s00394-018-1636-y. PMC 5959976. PMID 29541907. ^ Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L (2017). "Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials". Diabetes Research and Clinical Practice. 131: 124–131. doi:10.1016/j.diabres.2017.07.006. PMID 28750216. ^ a b American Diabetes Association Professional Practice Committee (2019). "Professional Practice Committee: Standards of Medical Care in Diabetes—2019". Diabetes Care. 42 (Supplement 1): s46–s60. doi:10.2337/dc19-S005. PMID 30559231. ^ Seckold R, Fisher E, de Bock M, King BR, Smart CE (2019). "The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: a review of clinical outcomes". Diabet. Med. (Review). 36 (3): 326–334. doi:10.1111/dme.13845. PMID 30362180. S2CID 53102654. ^ a b c "Top 5 worst celeb diets to avoid in 2018". British Dietetic Association. December 7, 2017. Retrieved December 1, 2020. The British Dietetic Association (BDA) nowadays revealed its much-anticipated annual record of superstar diets to steer clear of in 2018. The line-up this 12 months includes Raw Vegan, Alkaline, Pioppi and Ketogenic diets in addition to Katie Price's Nutritional Supplements. ^ a b Maughan, Ron (June 2013). "Surgery Oxford". www.onesearch.cuny.edu. ^ Mehta S (October 9, 2013). "Energetics of Cellular Respiration (Glucose Metabolism)". Biochemistry Notes, Notes.Further reading
"Compolition of foods raw, processed, prepared" (PDF). United States Department of Agriculture. September 2015. Retrieved October 30, 2016.External links
Wikimedia Commons has media associated with Carbohydrates. Wikiquote has quotations related to: CarbohydrateCarbohydrates, together with interactive models and animations (Requires MDL Chime) IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature Carbohydrates detailed Carbohydrates and Glycosylation – The Virtual Library of Biochemistry, Molecular Biology and Cell Biology Functional Glycomics Gateway, a collaboration between the Consortium for Functional Glycomics and Nature Publishing GroupvteMetabolism, catabolism, anabolismGeneral Metabolic pathway Metabolic community Primary dietary groupsEnergy metabolismAerobic respiration Glycolysis → Pyruvate decarboxylation → Citric acid cycle → Oxidative phosphorylation (electron transport chain + ATP synthase)Anaerobic respiratory Electron acceptors are other than oxygenFermentation Glycolysis → Substrate-level phosphorylation ABE Ethanol Lactic acidSpecific pathsProtein metabolism Protein synthesis CatabolismCarbohydrate metabolism(carbohydrate catabolismand anabolism)Human Glycolysis ⇄ Gluconeogenesis Glycogenolysis ⇄ Glycogenesis Pentose phosphate pathway Fructolysis Galactolysis Glycosylation N-linked O-linkedNonhuman Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation Xylose metabolism RadiotrophismLipid metabolism (lipolysis, lipogenesis)Fatty acid metabolism Fatty acid degradation (Beta oxidation) Fatty acid synthesisOther Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse ldl cholesterol shippingAmino acid Amino acid synthesis Urea cycleNucleotide metabolism Purine metabolism Nucleotide salvage Pyrimidine metabolismOther Metal metabolism Iron metabolism Ethanol metabolism vteFood chemistry Additives Carbohydrates Coloring Enzymes Essential fatty acids Flavors Fortification Lipids "Minerals" (Chemical components) Proteins Vitamins Water vteTypes of carbohydratesGeneral Aldose Ketose Furanose PyranoseGeometry Anomer Cyclohexane conformation MutarotationMonosaccharidesDioses Aldodiose GlycolaldehydeTrioses Aldotriose Glyceraldehyde Ketotriose DihydroxyacetoneTetroses Aldotetroses Erythrose Threose Ketotetrose ErythrulosePentoses Aldopentoses Arabinose Lyxose Ribose Xylose Ketopentoses Ribulose Xylulose Deoxy sugars DeoxyriboseHexoses Aldohexoses Allose Altrose Galactose Glucose Gulose Idose Mannose Talose Ketohexoses Fructose Psicose Sorbose Tagatose Deoxy sugars Fucose Fuculose RhamnoseHeptoses Ketoheptoses Mannoheptulose SedoheptuloseAbove 7 Octoses Nonoses Neuraminic acidMultipleDisaccharides Cellobiose Isomaltose Isomaltulose Lactose Lactulose Maltose Sucrose Trehalose TuranoseTrisaccharides Maltotriose Melezitose RaffinoseTetrasaccharides StachyoseOtheroligosaccharides Acarbose Fructooligosaccharide (FOS) Galactooligosaccharide (GOS) Isomaltooligosaccharide (IMO) MaltodextrinPolysaccharides Beta-glucan Oat beta-glucan Lentinan Sizofiran Zymosan Cellulose Chitin Chitosan Dextrin / Dextran Fructose / Fructan Inulin Galactose / Galactan Glucose / Glucan Glycogen Hemicellulose Levan beta 2→6 Lignin Mannan Pectin Starch Amylopectin Amylose Xanthan gum Category Authority keep an eye on GND: 4164517-0 LCCN: sh85020080 MA: 2778977261 NDL: 00572696 Retrieved from "https://en.wikipedia.org/w/index.php?title=Carbohydrate&oldid=1015392316"Carbohydrates - Our food stores Carbohydrates always play ...

Digestion, Absorption, and Transport in Digestive System

Why is glycogen a complex carbohydrate? - Quora
Frozen Vegan Food By Plant Based Best Protein Burger From ...

Forming glycogen as energy storage in the liver is an ...

PPT - Plant Nutrients PowerPoint Presentation, free ...
Biological Molecules : Synthesis/Hydrolysis and Carbohydrates
PPT - Plant Nutrients PowerPoint Presentation, free ...
Biochemistry

PPT - CARBOHYDRATES PowerPoint Presentation, free download ...

Chemistry of carbohydrates polysaccharides part -3 homoglycans

Nutrients 2012

Carbohydrates Novosibirsk State Agrarian University ...

Carbohydrates - WOW! Macromolecules

PPT - Carbohydrates PowerPoint Presentation, free download ...
Are Starch Molecules Larger Than Glucose Molecules ...

Carbohydrates - Cellulose

PPT - Ch 3 Carbon Compounds PowerPoint Presentation, free ...

Starch - Chemistry LibreTexts

Hydrolysis Reactions Dehydration Hydrolysis Synthesis ...

PPT - Carbohydrates PowerPoint Presentation, free download ...






No comments:
Post a Comment